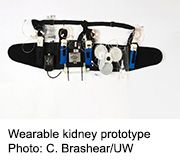
Small trial suggests device might be feasible, potentially freeing patients from dialysis machines
WEDNESDAY, June 8, 2016 (HealthDay News) — A wearable artificial kidney may be an upcoming option for dialysis patients, according to a proof-of-concept study published online June 2 in JCI Insight.
A prototype of such a device was recently tested on seven patients at the University of Washington Medical Center in Seattle. The study was led by the device’s inventor, Victor Gura, M.D., of the Cedars-Sinai Medical Center in Los Angeles and chief medical officer of Blood Purifications Technologies in California. The trial was designed to see how well the wearable kidney might work to safely take over some of the functions of failed kidneys. Patients used the device for up to 24 hours.
The device successfully cleared the blood of urea, creatinine, and phosphorus. Patients seemed to tolerate the therapy well, with no effect on circulation and no serious adverse effects, Gura’s team found.
Overall, the research team believes that a wearable artificial kidney is feasible. However, the researchers said some redesigns are needed to correct device-related technical problems that occurred during testing. For example, there was excessive formation of carbon dioxide gas bubbles in the dialysis solution, and intermittent variations in solution and blood flow, Gura’s team explained. The device redesigns will also focus on ease of use and reliability during use, because the objective is to enable patients to undergo dialysis at home.
The study was funded by the Wearable Artificial Kidney Foundation and Blood Purification Technologies.
Full Text
Copyright © 2016 HealthDay. All rights reserved.