Tag: Side Effects
For U.S. Teens, Most Adverse Events to COVID-19 Vaccine Nonserious
Frequency of local, systemic reactions reported for teens enrolled in v-safe similar to preauthorization clinical trials
Review: Benefit Outweighs Harm of Statins for Primary Prevention of CVD
Risk for adverse events attributable to statins low when used for primary prevention of cardiovascular disease
FDA Adds Guillain-Barré Warning to J&J COVID-19 Vaccine
About 100 preliminary reports of Guillain-Barré have been detected after 12.8 million doses of Johnson & Johnson vaccine were administered
Venous Thromboembolic Event Rates Up With ChAdOx1-S Vaccine
However, absolute risks are small, with 11 excess venous thromboembolic events per 100,000 vaccinations
Systemic Reactogenicity Up With Heterologous COVID-19 Vaccine Doses
More reported feverishness, chills, fatigue, headache, joint pain, malaise, muscle ache after heterologous versus homogenous doses
ACC: Common Medications Can Raise Blood Pressure
Nearly one in five adults with hypertension take medications that can increase blood pressure, such as antidepressants, NSAIDs, oral steroids
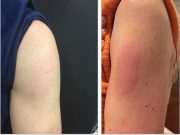
Delayed Hypersensitivity Reaction to Moderna COVID-19 Vaccine ID’d
Reaction occurred at or near injection site; reactions were pruritic, painful, and edematous pink plaques
Guidance Issued for Managing CVST After COVID-19 Vaccination
CVST with thrombocytopenia reported after COVID-19 vaccine with adenoviral vector; management similar to heparin-induced thrombocytopenia
12 Cases of CVST Reported for Ad26.COV2.S Vaccine in the U.S.
Time from vaccination to symptom onset varied from six to 15 days; initial presentation was headache in 11 cases
Side Effects of BNT162b2, ChAdOx1 nCoV-19 Vaccines Quantified
Systemic side effects for 13.5 and 22.0 percent after first, second dose of BNT162b2; 33.7 percent after first dose of ChAdOx1 nCoV-19