Tag: Gene Therapy
Sarepta’s Gene Therapy Under Scrutiny After Patient Death
FDA Approves Kebilidi for Aromatic L-Amino Acid Decarboxylase Deficiency
Approval represents first gene therapy for treatment of AADC deficiency
Lentiviral Gene Therapy Beneficial for Early Cerebral Adrenoleukodystrophy
No major functional disabilities observed at month 24; 81 percent of patients had no major functional disabilities at median of six years
Hematologic Cancer Develops in Some Patients Receiving Eli-Cel
Hematologic cancer developed in seven of 67 patients with cerebral adrenoleukodystrophy receiving eli-cel gene therapy
Fidanacogene Elaparvovec Superior to Prophylaxis for Hemophilia B
Adeno-associated virus gene-therapy vector for hemophilia B superior for reducing annualized bleeding rate
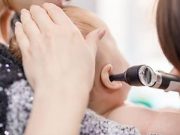
Bilateral Gene Therapy Safely Restores Hearing in Children Born Deaf
Bilateral hearing restoration seen for all five patients; speech perception and capability of sound source localization restored
Breakthrough Gene Therapy Enables Infant Born Deaf to Hear
Child's hearing and speech are currently on track, parents and doctors say
First Gene Therapy for Children With Metachromatic Leukodystrophy Approved by FDA
Approval is for a single-dose gene therapy infusion
Gene Therapy for Sickle Cell Disease Likely Cost-Effective at <$2 Million
Incremental cost-effectiveness ratios were $126,000 and $281,000 per QALY for two simulation models from a societal perspective
First Gene-Editing Therapies for Sickle Cell Disease Approved by FDA
In both therapies, stem cells are removed from a patient's blood for treatment