Tag: Food & Drug Administration
FDA Requires Safety Label Changes for Fluoroquinolones
Changes will describe hypoglycemic coma and clarify and identify mental health side effects
High Rates of Salmonella Contamination ID’d in Kratom
As of end of May 2018, 199 cases of salmonellosis ID'd that were linked to kratom consumption
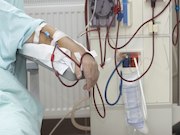
FDA Permits Marketing of Devices to Create Arteriovenous Fistula
Two devices designed to create arteriovenous fistula in patients with CKD in need of hemodialysis
FDA Approves Epidiolex for Severe Forms of Epilepsy
Cannabidiol oral solution approved for patients 2 and older with Lennox-Gastaut and Dravet syndromes
FDA Approves Continuous Glucose Monitoring System
Eversense CGM, which has fully implantable sensor to detect glucose, approved for adults with diabetes
Many Drugs Made Available Via FDA Expanded Access Programs
Half of meds ID'd in expanded access programs treat cancer; others for metabolic, endocrine diseases
FDA Approves First Generic Under-the-Tongue Suboxone
May only be prescribed by Drug Addiction Treatment Act-certified prescribers
FDA Warns Websites Marketing Unapproved Opioids
As part of comprehensive effort to target online sales, networks operating 53 sites received warnings
FDA OKs 1st Biosimilar to Prevent Chemo-Related Infections
Fulphila, biosimilar to Neulasta, helps reduce risk of infection during cancer treatment
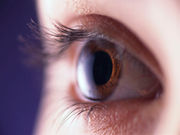
FDA Approves First Artificial Iris
CustomFlex Artificial Iris improves light sensitivity and glare, cosmetic appearance of the eye