Tag: Food & Drug Administration
FDA Reviewers Give Thumbs Down to New ALS Drug
Agency said results were 'not persuasive,' citing missing data, mistakes in enlisting patients, other issues
FDA Says Gene-Edited Cattle Are Safe to Eat
Cattle are the third genetically altered animals approved for human consumption after salmon and pigs
FDA Expands Approval of Jardiance for Patients With Heart Failure
Jardiance received approval to reduce the risk for cardiovascular death and hospitalization for patients with heart failure
FDA Approves Marketing of First Condom Designed for Anal Sex
Condom was approved through the De Novo premarket review pathway for low- to moderate-risk devices of a new type
FDA Warns of Rising Dangers of Unapproved Drug Tianeptine
Possible risks from taking the drug include accidental poisoning and addiction
FDA Warns of Infant Formula Powders Tied to Infections
Agency is investigating three Cronobacter sakazakii infections and one Salmonella Newport infection among four infants
U.S. Senate Narrowly Confirms Califf to Head FDA
Califf briefly served as FDA commissioner toward the end of President Barack Obama's administration
Many COVID-19 Home Test Users May Act on Quarantine Inappropriately
Users may fail to quarantine or quarantine unnecessarily because of misinterpretation of pretest probability
FDA Panel Rejects Lilly Cancer Drug Tested Only in China
Drug was to be used alongside chemotherapy for patients with metastatic non-small cell lung cancer
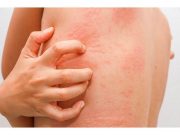
FDA Approves Rinvoq for Treatment of Atopic Dermatitis
Approval indicated for those 12 years and older with atopic dermatitis that has not responded to previous treatment