Tag: Drug Approvals
FDA Approves Siliq for Plaque Psoriasis
Injected medication approved for use by patients who've failed other treatments
FDA Approves Emflaza for Duchenne Muscular Dystrophy
Approved to treat Duchenne muscular dystrophy in patients 5 years and older
FDA Approves Newborn Screening Tests for Metabolic Disorders
Screens used to detect Mucopolysaccharidosis Type 1, Pompe, Gaucher, and Fabry
FDA Approves Trulance for Chronic Idiopathic Constipation
Drug designed to stimulate secretion of intestinal fluid
FDA Grants Fast-Track Approval to Ovarian Cancer Drug
Rubraca's use is specific to women with deleterious BRCA mutations
FDA OKs Autologous Cellularized Scaffold for Knee Cartilage Repair
For symptomatic, full-thickness cartilage defects of the knee in adult patients
FDA Approves Intrarosa for Postmenopausal Pain During Sex
Drug contained in a once-daily vaginal insert
FDA Approves Device to Prevent Recurrent Strokes in PFO Patients
For patients who had prior stroke related to patent foramen ovale
FDA Approves New Treatment for Advanced Soft Tissue Sarcoma
Sanctioned for use with doxorubicin in cases that cannot be treated with radiation or surgery
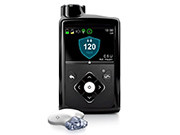
FDA Approves ‘Artificial Pancreas’ for Type 1 Diabetes
MiniMed 670G hybrid closed loop system automatically monitors glucose, delivers insulin