Tag: Drug Approvals
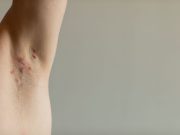
FDA Approves Bimzelx for Hidradenitis Suppurativa
Approval based on phase 3 trials showing clinical response at 16 weeks, with sustained benefit at 48 weeks
FDA Approves Cobenfy for Adults With Schizophrenia
Approval marks first new class of treatment in decades and the first muscarinic agonist
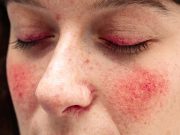
FDA Approves Emrosi for Rosacea in Adults
Phase 3 trials show significant reduction in total lesion count versus Oracea and placebo
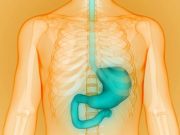
FDA Approves Vyloy for Advanced Gastric or Gastroesophageal Junction Cancer
Vyloy is the first and only CLDN18.2-targeted therapy approved in the United States
FDA Approves Vyalev for Advanced Parkinson Disease
Vyalev is the first and only subcutaneous 24-hour continuous infusion of levodopa-based therapy for treatment of motor fluctuations
FDA Approves Dupixent for Chronic Obstructive Pulmonary Disease
Approval is the first in the U.S. for a biologic for the condition
FDA Approves Miplyffa for Treatment of Niemann-Pick Disease, Type C
Miplyffa is the first drug approved for treating associated neurological symptoms in adults and children 2 years and older
FDA Approves Bimzelx for Three New Indications
New indications include active psoriatic arthritis, nonradiographic axial spondyloarthritis with objective signs of inflammation, and ankylosing spondylitis
FDA Approves Injectable Ocrevus Zunovo for Relapsing, Progressive MS
Phase 3 data show efficacy and safety profile similar to intravenous administration
FDA Approves Ebglyss for Moderate-to-Severe Atopic Dermatitis
Approval is for new, first-line biologic treatment of eczema that is not well controlled with topicals in people 12 years and older