Tag: Clinical Trials
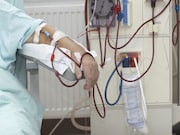
Trial Results Unclear for Benefit of Longer Hemodialysis Sessions
Intervention uptake not sufficient to assess impact of longer hemodialysis sessions on patient outcomes
Genetic Research Results May Warrant Updates to Participants
Participants should be recontacted in some cases of new information about variant, newly ID'd variants
ACC: Robust Evidence Lacking for Many Cardiovascular Guidelines
Few recommendations in cardiovascular society guidelines supported by evidence from multiple RCTs
Influences on New NIH Policy for Age Limits in Research Explored
'Inclusion Across the Lifespan' policy eliminates non-risk-justified age limits on research participants
Structural, Clinical Barriers Prevent Cancer Trial Participation
Trial unavailable at patients' institution 55.6 percent of the time; 21.5 percent of patients ineligible
Many Systematic Reviews Do Not Fully Report Adverse Events
Of 146 protocols analyzed, 65 percent fully reported adverse events as intended by the protocol
Comorbidities Adversely Linked to Cancer Trial Participation
Reduction in trial discussions, trial offers, trial participation seen with one or more comorbidities
Few Mandatory Pediatric Postmarketing Studies Completed
33.8 percent of mandatory pediatric postmarketing studies completed during 6.8 years of follow-up
Financial Conflicts of Interest Prevalent Among CPG Authors
Two studies show high prevalence among authors of guidelines on high-dollar meds, in gastroenterology
Barriers to Prostate Cancer Research in Black Men Identified
Mistrust, lack of knowledge, unfavorable attitudes are barriers to genomic testing, study participation