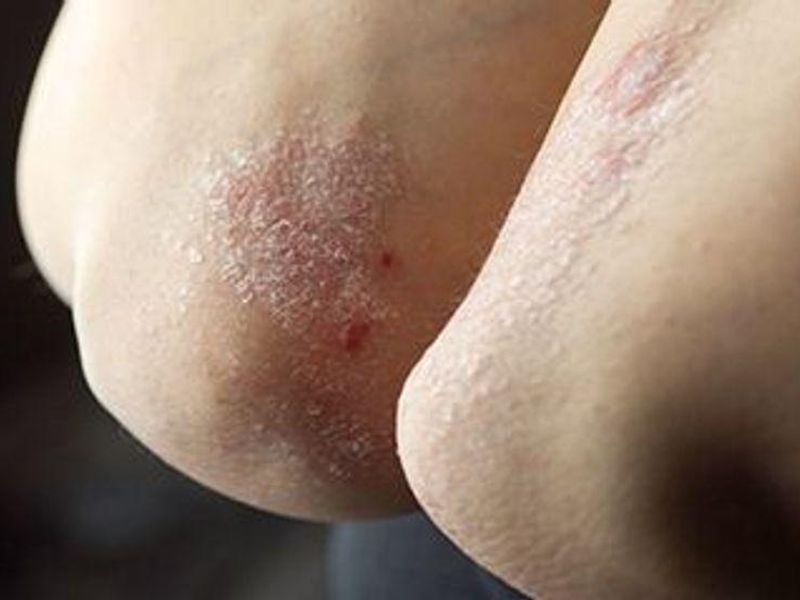
In phase 2 trial, spesolimab cleared more lesions than placebo, but it was associated with infections, systemic drug reactions
MONDAY, Dec. 27, 2021 (HealthDay News) — At one week, the incidence of lesion clearance was higher with spesolimab than placebo among patients with generalized pustular psoriasis (GPP), according to a study published in the Dec. 23 issue of the New England Journal of Medicine.
Hervé Bachelez, M.D., Ph.D., from the Assistance Publique-Hôpitaux de Paris Hôpital Saint-Louis, and colleagues randomly assigned 53 patients with a GPP flare to receive a single dose of either spesolimab or placebo (35 and 18 patients, respectively). In both groups, patients could receive an open-label dose of spesolimab on day 8, an open-label dose of spesolimab as rescue medication after day 8, or both, and they were followed to 12 weeks. The primary end point was a Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) pustulation subscore of 0 to 4 (no visible pustules to severe pustulation) at week 1.
The researchers found that overall, 54 percent of patients in the spesolimab group and 6 percent in the placebo group had a pustulation subscore of 0 at week 1. Forty-three and 11 percent had a GPPGA total score of 0 or 1 in the spesolimab and placebo groups, respectively. Infections occurred in 17 percent of those assigned to spesolimab through the first week; at week 12, infections had occurred in 47 percent of those who received spesolimab at any time in the trial. Forty-six percent of those who received at least one dose of spesolimab had antidrug antibodies.
“Intravenous spesolimab resulted in higher rates of clearance of pustular lesions at one week than placebo but was associated with infections and systemic reactions,” the authors write.
Several authors disclosed financial ties to Boehringer Ingelheim, which manufactures spesolimab and funded the study.
Copyright © 2021 HealthDay. All rights reserved.