74 percent of respondents unaware of FDA-approved treatments; 56 percent said these options had not been discussed by clinician
By Elana Gotkine HealthDay Reporter
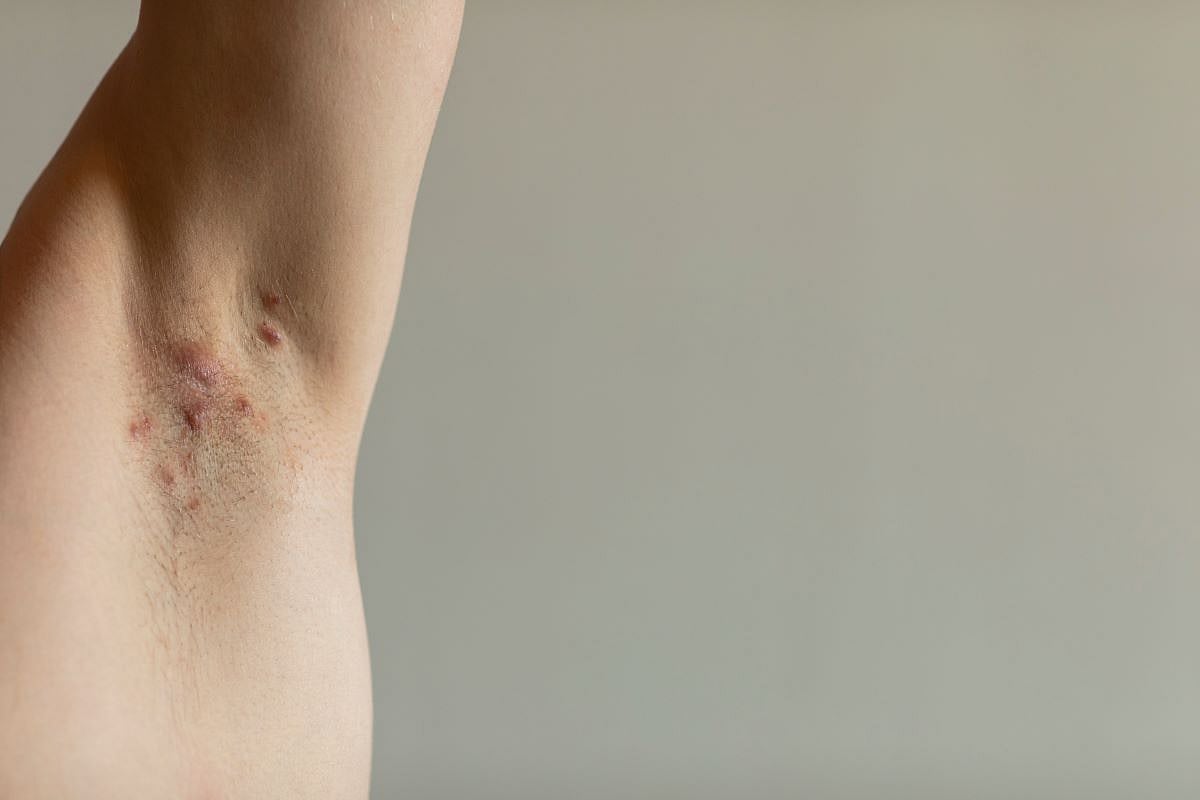
MONDAY, April 7, 2025 (HealthDay News) — The rates of respondent dissatisfaction with current hidradenitis suppurativa (HS) treatment options are high, likely due to undertreatment with available therapies, according to study published in the April issue of the Journal of Drugs in Dermatology.
Andeulazia Murdock, from the George Washington School of Medicine and Health Sciences in Washington, D.C., and colleagues distributed a survey to examine perceptions of current and emerging HS treatments and their impact on quality of life among respondents aged 18 years and older. Overall, 423 participants completed the entire survey.
The researchers found that <20 percent of respondents (76/423) were satisfied or very satisfied with current treatment options. Only 39 and/or 26 percent of the 244 patients with self-reported disease severity of Hurley stage II or III were being treated with biologics and/or hormone therapy, respectively, indicating undertreatment per current guidelines. Seventy-four percent of the respondents were unaware of U.S. Food and Drug Administration-approved treatments; more than half (56 percent) reported that these options had not been discussed by their dermatologist or health care provider. Sixty percent of respondents felt it was important to have FDA-approved therapies, believing it would result in improved physical health, mental health, and/or personal relationships (86, 78, and 60 percent, respectively).
“Our goal with this study was to highlight the multifaceted positive impact of new FDA treatments on those living with hidradenitis and the importance of pharma investment in this space,” coauthor Adam Friedman, M.D., also from the George Washington School of Medicine and Health Sciences, said in a statement. “We also found that there are still significant gaps in care and dissatisfaction with current treatment approaches.”
Copyright © 2025 HealthDay. All rights reserved.