Home 2015
Archives
FDA: Watch for Ketoacidosis With SGLT2 Inhibitors
Class of drugs includes canagliflozin, dapagliflozin, and empagliflozin
CDC: Untreated Swimming Water Can Foster Norovirus
Safe hygiene practices can prevent norovirus outbreaks, CDC says
CDC: Rates of ADHD Diagnoses Essentially Unchanged
Unchanged since 2007; boys still twice as likely to have the condition as girls
Tramadol-Related ER Visits Up 2005 to 2011
U.S. report found problem was greatest among women and patients 55 and older
CDC: Some Progress Seen in Foodborne Illness
But some types of foodborne bacteria, including Campylobacter and Vibrio, are on the rise
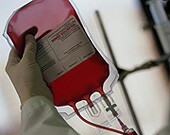
FDA Proposes Lifting Ban on Homosexual Blood Donations
Agency collecting comments on proposal for 60 days before issuing final rules
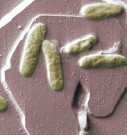
FDA Approves Avelox for Treatment of Plague
Yersinia pestis bacterium could be released intentionally in bioterrorism
FDA: Senza Device Relieves Spinal Pain Without Paresthesia
Implanted device treats chronic back pain
CDC: Mortality Rate From Falls Up for U.S. Seniors
Researchers also report that car crashes cause 1 in 7 unintentional deaths in older Americans
Medical Students Want to Focus Learning on Preparing for Future
Desired topics include leadership training, health policy, health economics, experiential learning