Home 2016
Archives
FDA Gives Tentative Approval to Tests of Gene-Modified Mosquitos
Agency says testing the insects in Florida Keys poses little risk to people, animals, the environment
Evidence Links Agent Orange to Bladder Cancer, Hypothyroidism
Also, veterans with Parkinson's-like symptoms can file claim for the condition
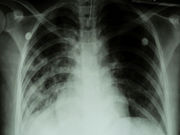
FDA Approves Xalkori for Rare Genetic Form of Lung Cancer
Drug approval expanded to include patients with ROS1-positive NSCLC
CDC: Zika Poses Serious Threat to Puerto Rico
Funds needed to battle the virus, which has been linked to severe birth defects
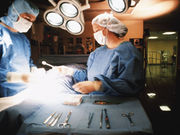
Complications Led to Loss of Organ in U.S. Uterus Transplant
Doctors at Cleveland Clinic aren't yet revealing what went wrong
USPSTF Urges Screening for Adults at High Risk of Latent TB
Grade B recommendation issued in draft recommendation statement on latent TB infection
Surgeons Offer Details on First U.S. Uterus Transplant
Patient will have to wait a year before she can try to become pregnant, Cleveland Clinic doctors say
CDC: Hospitals Making Progress Against Antibiotic Resistance
But hundreds of thousands of patients are still affected each year
Bloodstream Infection Outbreak in Wisconsin Linked to 18 Deaths
All patients were older than 65 and had serious underlying health conditions
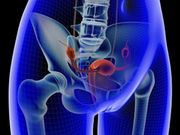
Ovarian CA Encompasses More Than One Kind of Malignancy
Better understanding needed to improve prevention, detection, treatment, U.S. panel notes