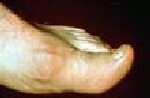
Classification criteria, which include clinical, lab, imaging domains have high sensitivity, specificity
WEDNESDAY, Sept. 30, 2015 (HealthDay News) — New classification criteria have been developed with high specificity and sensitivity for gout, according to an article published in the October issue of Arthritis & Rheumatology.
Tuhina Neogi, M.D., Ph.D., from the Boston University School of Medicine, and colleagues developed new classification criteria for gout. They conducted a systematic review of the literature on advanced imaging for gout, a diagnostic study with monosodium urate monohydrate (MSU) crystals as the gold standard, a ranking exercise of paper patient cases, and an exercise in multicriterion decision analysis. The classification criteria were tested in an independent data set.
The researchers reported that the entry criterion for the new classification criteria required the occurrence of one or more episodes of peripheral joint or bursal swelling, pain, or tenderness. The presence of MSU crystals in a symptomatic joint/bursa or in a tophus was considered to be sufficient for classification of a patient with gout, without need for further scoring. The new classification criteria included clinical, laboratory, and imaging domains. High sensitivity and specificity were seen for the criteria (92 and 89 percent, respectively).
“The new classification criteria, developed using a data-driven and decision analytic approach, have excellent performance characteristics and incorporate current state-of-the-art evidence regarding gout,” the authors write.
Several authors disclosed financial ties to the pharmaceutical industry.
Copyright © 2015 HealthDay. All rights reserved.