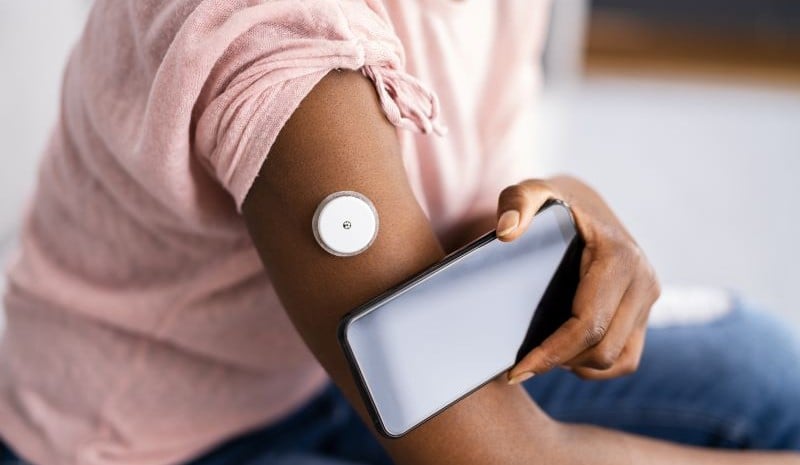
Dexcom Stelo Glucose Biosensor System is intended for people aged 18 years and older who have T2D but do not take insulin
By Physician’s Briefing Staff HealthDay Reporter
WEDNESDAY, March 6, 2024 (HealthDay News) — The first over-the-counter continuous glucose monitor (CGM) has been cleared for marketing by the U.S. Food and Drug Administration.
The new Dexcom Stelo Glucose Biosensor System, which will be available by summer, is intended for people 18 years and older who have type 2 diabetes but do not take insulin, according to the agency. The agency warned that the new system is not intended for patients with problematic hypoglycemia, noting it was “not designed to alert the user to this potentially dangerous condition.”
More than 25 million Americans have type 2 diabetes and do not use insulin, Dexcom noted. While the company’s G7 CGM system is available to these patients, they have to get a prescription for it. That changes with the arrival of an over-the-counter version of the tracking system.
The sensor will be worn on the upper arm, and it lasts for up to 15 days before it needs to be replaced, according to the Dexcom website.
Data from a clinical study provided to the FDA showed the device performed similarly to other integrated CGMs. Adverse events reported in the study included local infection, skin irritation, and pain or discomfort.
“CGMs can be a powerful tool to help monitor blood glucose. Today’s clearance expands access to these devices by allowing individuals to purchase a CGM without the involvement of a health care provider,” Jeff Shuren, M.D., J.D., director of the FDA Center for Devices and Radiological Health, said in an agency news release. “Giving more individuals valuable information about their health, regardless of their access to a doctor or health insurance, is an important step forward in advancing health equity for U.S. patients.”
Copyright © 2024 HealthDay. All rights reserved.