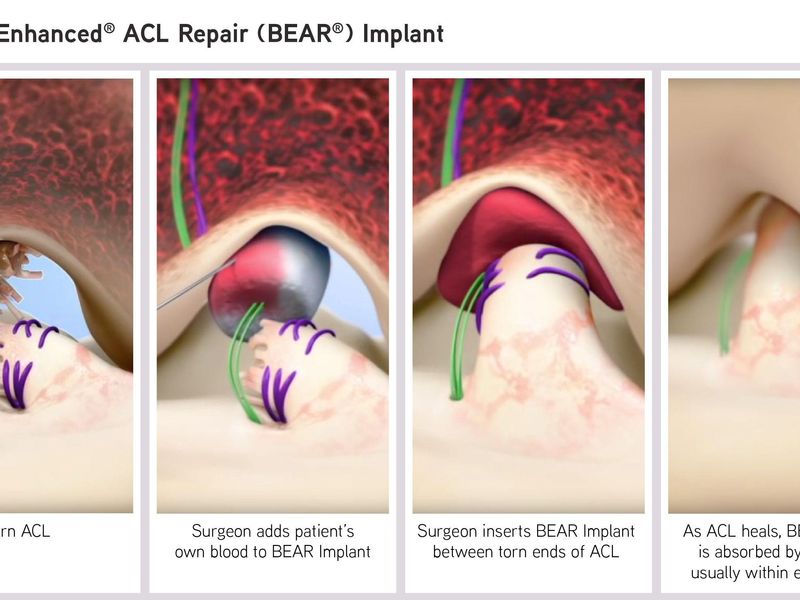
The BEAR Implant offers alternative to ACL reconstruction with allograft, autograft, or suture-only repair
THURSDAY, Dec. 17, 2020 (HealthDay News) — An anterior cruciate ligament (ACL) implant that offers an alternative to traditional ACL reconstruction has received marketing authorization from the U.S. Food and Drug Administration — the first approval for an ACL tear treatment in more than 30 years, the agency announced yesterday.
The Bridge-Enhanced ACL Repair (BEAR) Implant received approval under the De Novo premarket review pathway, the FDA regulatory pathway for low- to moderate-risk devices of a new type. The resorbable implant, made from bovine collagen, is the only currently available alternative to reconstruction with allograft, autograft, or suture-only repair for an ACL rupture. The approval is indicated for skeletally mature patients aged at least 14 years old who have a complete ACL rupture confirmed on magnetic resonance imaging. The ACL stump must be attached to the tibia to construct the repair.
The surgeon secures the BEAR Implant via suture and injects the patient’s own blood into the implant to form a device-protected clot thereby enabling the body’s healing process. According to the FDA, within about eight weeks of the procedure, the implant is absorbed and replaced by the body’s own tissue.
Approval of the BEAR Implant was based on data from the BEAR II clinical trial, showing noninferiority of the implant to ACL reconstruction in 14- to 35-year-old patients. In the randomized, controlled trial of 100 patients with complete ACL rupture, 65 patients received the BEAR Implant and 35 patients in a control group underwent ACL reconstruction with autograft. At two years, average International Knee Documentation Committee Subjective Scores were 88.6 and 84.6 in the BEAR Implant and control groups, respectively. In arthrometric assessments at two years, patients who received the BEAR Implant had an average laxity in the treated knee that was 1.7 mm greater than that observed in the untreated knee, while control patients had an average 1.8-mm greater laxity.
Marketing authorization was granted to Miach Orthopaedics Inc. The company says it plans to conduct a limited market release of the implant in early 2021.
Copyright © 2020 HealthDay. All rights reserved.