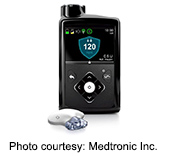
MiniMed 670G hybrid closed loop system automatically monitors glucose, delivers insulin
THURSDAY, Sept. 29, 2016 (HealthDay News) — The first automated insulin delivery device for type 1 diabetes has been approved by the U.S. Food and Drug Administration for patients aged 14 and older.
Often called an “artificial pancreas,” the MiniMed 670G hybrid closed loop system automatically monitors glucose levels every five minutes and delivers insulin when needed with little or no input from the user, the FDA said in a news release.
Clinical testing of the device involved 123 patients with type 1 diabetes. No serious adverse reactions were reported. But risks associated with the device include hyperglycemia, hypoglycemia, and skin irritation near where the device’s sensor attaches to the body, the FDA said. The product should be considered unsafe for use by children aged 6 or younger and for patients who require fewer than eight units of insulin daily.
As a condition of approval, manufacturer Medtronic is required to conduct a post-market study to evaluate “how the device performs in real-world settings,” the FDA said. In addition, Medtronic, based in Dublin, is studying the device’s safety and effectiveness in children aged 7 to 13.
Health News Copyright © 2016 HealthDay. All rights reserved.