FDA Approves Nuplazid for Parkinson’s Hallucinations
Half of patients with Parkinson's may experience hallucinations, delusions
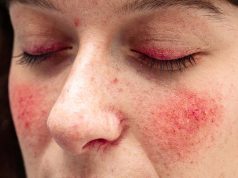
Guidelines Developed for Management of Atopic Dermatitis
Current treatment strategies include use of drug therapy, skin care, avoiding exacerbating factors
FDA Reconsidering Training for Doctors Prescribing Opioids
Next week, FDA committee of outside experts will meet to review risk-management plans
Generic Crestor Approved by FDA
First generic version of Crestor (rosuvastatin calcium)
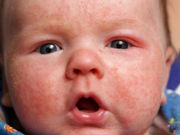
Preventive Topical Steroids Cut Atopic Dermatitis Severity
May also prevent an increase in aeroallergen-specific IgE levels
Review Compares Metformin, OCPs for Teens With PCOS
Four randomized controlled trials show benefits for metformin and oral contraceptive pills
Ixazomib Ups Progression-Free Survival in Multiple Myeloma
Up in all prespecified patient groups, including those with high-risk cytogenetic abnormalities
Article Discusses Workplace Violence in Health Care
Workplace violence often underreported; health care sector among industries most subject to violence
Atomoxetine Use Doesn’t Up Suicide Risk in Children
No increased risk for current atomoxetine use in first- and second-line treatment of youths
Simvastatin/Ezetimibe Not Beneficial in Alopecia Areata
In sample of 20 patients, most report no hair regrowth following six months of treatment