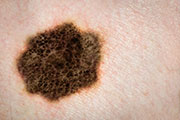
Second study supports new immunotherapy for enhanced survival in advanced melanoma
MONDAY, June 1, 2015 (HealthDay News) — Not all melanoma patients need surgery to remove lymph nodes surrounding their tumor, according to findings scheduled for presentation Saturday at the annual meeting of the American Society of Clinical Oncology, held from May 29 to June 2 in Chicago. A second study presented Sunday and published simultaneously in the New England Journal of Medicine indicates that new immunotherapy could significantly extend life expectancy in advanced melanoma.
Claus Garbe, M.D., a professor of dermatology at the University of Tubingen in Germany, and colleagues included 483 patients with stage III melanoma and a positive lymph node biopsy who had their primary tumor removed. The patients were then randomly assigned to observation only or complete lymph node dissection (CLND).
During follow-up, in which half the patients were monitored for more than three years, the cancer had spread in 14.6 percent of patients in the observation group, compared with 8.3 percent in the CLND group. However, no statistically significant improvement in survival occurred between the two groups at three years or five years, the researchers reported.
In the second study, researchers randomly assigned 945 patients with previously untreated, advanced melanoma to receive ipilimumab (Yervoy), nivolumab (Opdivo), or a combination of the two. The researchers found that nivolumab slowed cancer progression in melanoma patients by more than double, compared with ipilimumab (6.9 versus 2.9 months). The delay in cancer progression nearly quadrupled when the two drugs were combined, compared with taking ipilimumab alone (11.5 versus 2.9 months). The combination therapy did demonstrate greater side effects; however, the researchers noted that no deaths occurred during the large-scale, international clinical trial.
The second study was supported by Bristol-Myers Squibb, the manufacturer of nivolumab.
Copyright © 2015 HealthDay. All rights reserved.