ULT strongly recommended for tophaceous gout, radiographic damage due to gout, gout flares
THURSDAY, May 21, 2020 (HealthDay News) — In an updated American College of Rheumatology guideline, published online May 11 in Arthritis Care & Research, recommendations are presented for the management of gout.
John D. FitzGerald, M.D., Ph.D., from the University of California in Los Angeles, and colleagues conducted a systematic literature review to provide guidance for the management of gout. A total of 42 recommendations were generated, including 16 strong recommendations.
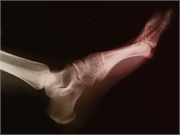
The authors note that the strong recommendations include urate-lowering therapy (ULT) initiation for all patients with tophaceous gout, radiographic damage due to gout, or frequent gout flares. The preferred first-line ULT is allopurinol, including for those with moderate-to-severe chronic kidney disease. A low starting dose of allopurinol (≤100 mg/day or lower in the case of chronic kidney disease) or febuxostat (≤40 mg/day) should be used. In addition, a treat-to-target management strategy should be employed, with titration of ULT guided by serial serum urate measurements, targeting a serum urate of <6 mg/dL. Concomitant anti-inflammatory prophylaxis therapy is strongly recommended when initiating ULT for a duration of at least three to six months. For patients of Southeastern Asian descent and for African-American patients, testing for the HLA-B*5801 allele is conditionally recommended prior to starting allopurinol. Colchicine, nonsteroidal anti-inflammatory drugs, or glucocorticoids are strongly recommended for the management of gout flares.
“As data continue to emerge supporting best practices in management, implementation of these recommendations will ideally lead to improved quality of care for patients with gout,” the authors write.
Several authors disclosed financial ties to the pharmaceutical industry.
Copyright © 2020 HealthDay. All rights reserved.