Brineura will be used to treat children with late infantile neuronal ceroid lipofuscinosis type 2 Batten
FRIDAY, April 28, 2017 (HealthDay News) — Brineura (cerliponase alfa) has been approved by the U.S. Food and Drug Administration to treat a specific form of Batten disease, a rare set of genetic disorders that typically begin between ages 2 and 4, the agency said in a news release.
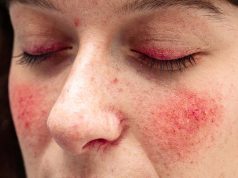
Initial symptoms of the late infantile neuronal ceroid lipofuscinosis type 2 (CLN2) form of Batten, for which the drug is approved, usually include language delay, epilepsy, and ataxia. Affected children also develop vision loss and myoclonus. Many require a wheelchair by late childhood and often don’t survive past their teens, the agency said. Batten is rare, occurring in as many as four of every 100,000 births in the United States.
Cerliponase alfa is an enzyme replacement drug, and its active ingredient is a recombinant form of the human TPP1 enzyme. It is administered directly into the cerebrospinal fluid through an intraventricular access device, the FDA said.
The efficacy of cerliponase alfa was evaluated in a nonrandomized, single-arm dose-escalation study involving 22 symptomatic pediatric patients with CLN2 disease who were compared with 42 untreated historical controls. The drug’s safety was evaluated in 24 children ages 3 to 8 with CLN2 disease. The most common adverse effects included fever, electrocardiogram abnormalities, vomiting, seizures, headache, and irritability. The drug hasn’t been evaluated in children under age 3, the FDA said. As a condition of approval, its manufacturer will be required to evaluate the drug in younger children and to study for a minimum of 10 years its long-term health effects.
Approval of Brineura was given to BioMarin Pharmaceutical, based in San Rafael, California
Health News Copyright © 2017 HealthDay. All rights reserved.